

When the valve is opened the reaction quickly goes to completion.
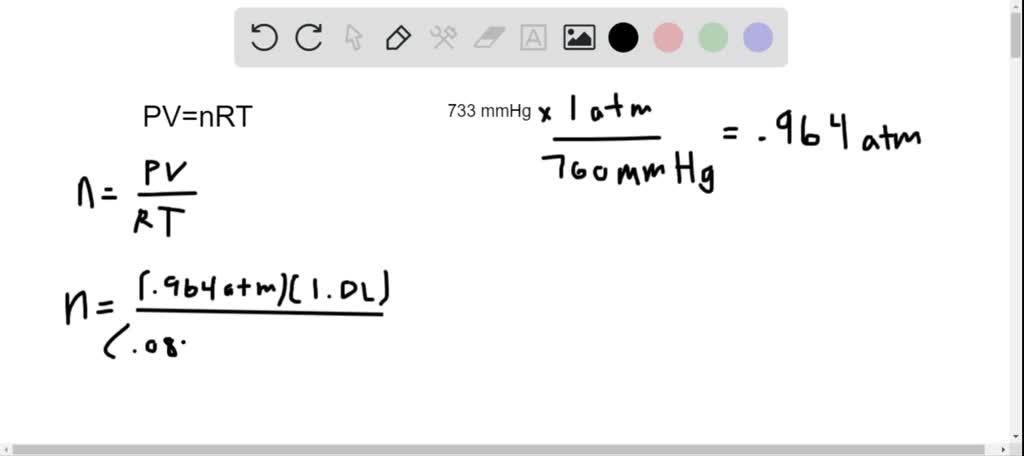
Nitric oxide reacts with oxygen gas as shown below: NO (g) + O (g) NO (g) Initially NO and O are separated as shown in the diagram below. What volume of hydrogen gas is produced in this reaction? (Vapor pressure of water at 5 C is 3.8 mmhg) Al (s) + 6 HCl (aq) AlCl3 (aq) + 3 H (g) H = atm - HO = 744 mmhg mmhg = 70. A 1.65-g sample of Al is reacted with excess HCl and the hydrogen produced is collected over water at 5 C at a barometric pressure of 744 mmhg.

atm and 6 C when 60 g of nitroglycerine is decomposed. Nitroglycerin, an explosive compound, decomposes according to the equation below: 4 C3H5(NO3)3 (s) 1 CO (g) + 10 HO (g) + 6 N (g) + O (g) Calculate the total volume of gases produced at 1. Life rafts and weather balloons can be inflated by the reaction shown below: CaH (s) + HO (l) Ca(OH) (aq) + H (g) How many grams of CaH are needed to produce 10.0 L of hydrogen gas at 740 mmhg and 3 C? (740 mmhg x )(10.0 L ) V 760 mmhg n H = mol RT (0.081 )(96 K ) mol K mol H CaH mol H x 4.10 g x = 8.43 g CaH 9. A mixture of 4.00 g of hydrogen and 10.0 g of helium are in a 4.30-L flask at 0 C. Calculate the density of HBr gas in g/l at 733 mmhg and 46 C. moles of gas = 0.80 L x.4 L = mol molar mass = g = 3.0 g/mol mol 6. How many grams of gas must be released to reduce the pressure in the cylinder to 1.15 atm if the temperature remains constant? Mass of gas present at 1.15 atm V n RT (1.15 atm )(34.0 L ) (0.081 )(95 K mol K mass of oxygen = mol Mass of gas that must be removed 305 g 51.6 g = 53 g = mol ) 3.0 g x = 51.6 g 1Ģ 5. A 34.0-L cylinder contains 305 g of oxygen gas at C. If the final pressure in the tank is 71 mmhg, what must have been the original pressure (in atm) in the cylinder? = x 1 V = (71 mmhg V 1 x 760 mmhg 1875 L ) x = 49.7 atm 35.8 L 4. A 35.8 L cylinder of Argon gas is connected to and transferred into an evacuated 1875-L tank at constant temperature. What is the volume of this gas at 1.30 atm and 0 C? V = V x 1 1 = 6.7 L x 75 mmhg = 0.3 L 760 mmhg 1.30 atm x 3. A sample of oxygen gas has a volume of 6.7 L at 75 mmhg and 0 C. atm = atm h = 5 cmhg gas atm 760 mmhg x = 741 mmhg 10 mmhg x 1 cmhg = 50 mmhg = - h = 741 mmhg - 50 mmhg = 1 mmhg. Determine the pressure of the gas (in mmhg) in the diagram below, given atmospheric pressure= atm. Volume to weight, weight to volume and cost conversions for Refrigerant R-401A, liquid (R140A) with temperature in the range of -51.12☌ (-60.016☏) to 68.34☌ (155.1 Chemistry 101 ANSWER KEY REVIEW QUESTIONS Chapter 5 1. Calculate how much of this gravel is required to attain a specific depth in a cylindrical, quarter cylindrical or in a rectangular shaped aquarium or pond List of these foods starting with the highest contents of Tocotrienol, gamma and the lowest contents of Tocotrienol, gamma Gravels, Substances and OilsĬaribSea, Marine, CORALine, Volcano Beach weighs 865 kg/m³ (54.00019 lb/ft³) with specific gravity of 0.865 relative to pure water. Millimeters of mercury to newtons per square meter conversion cardsĭECORATING ICING BLUE, UPC: 071169730022 weigh(s) 304 grams per metric cup or 10.2 ounces per US cup, and contain(s) 417 calories per 100 grams (≈3.53 ounces) ĥ50 foods that contain Tocotrienol, gamma.


 0 kommentar(er)
0 kommentar(er)
